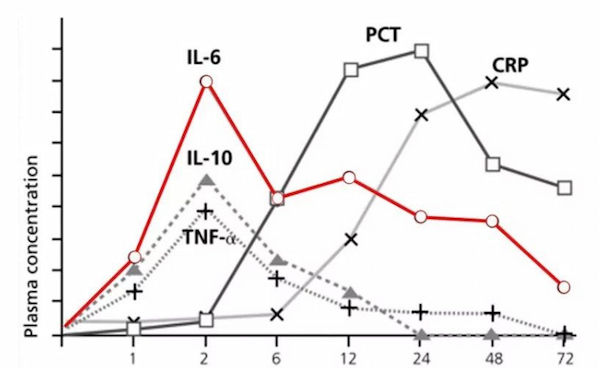
1. Regional Market Opportunities: Developing Countries and Primary Healthcare
Widespread availability of primary healthcare facilities
Pain points: Community hospitals and clinics in developing countries (such as India, Southeast Asia, and Africa) lack skilled nurses, resulting in high rates of failed intravenous punctures finder.
Opportunity: Promote low-cost portable devices (e.g., domestically produced models under $4,000) in conjunction with government healthcare upgrade programs.
Vaccination and Public Health
Demand: Global vaccination coverage is increasing, but vascular localization is challenging for children in remote areas.
Solution: Collaborate with the United Nations Children’s Fund (UNICEF) to develop durable, battery-powered models suitable for high-temperature and high-humidity environments.
II. Application Scenario Expansion: Non-Traditional Medical Fields
Aesthetic Medicine and Minimally Invasive Treatments
Applications:
Precise marking of vascular pathways before varicose vein treatment.
Avoiding dangerous veins (such as the “triangle area” of the nose) during hyaluronic acid injections.
Market: The global aesthetic medicine market grows by over 10% annually, with China projected to reach 300 billion yuan by 2025. Devices can be bundled for sale to private aesthetic medicine institutions.
Veterinary Market
Demand: The rise of pet healthcare, but animal vessels (e.g., cats, exotic pets) are finer and covered by fur.
Innovation: Adjusting infrared wavelengths to suit animal skin characteristics (e.g., dog vessels absorb 850nm more strongly).
Emergency and Disaster Response
Scenario: Rapidly establishing venous access in harsh environments such as battlefields or earthquakes.
Product Direction: Earthquake-resistant and dust-proof design.
III. Opportunities Driven by Technological Innovation
AI + Augmented Reality (AR)
Function:
Real-time prediction of blood vessel depth and direction.
Combined with smart glasses to achieve “hands-free operation” (suitable for sterile operating room environments).
Barriers: Need to overcome algorithm adaptability for dark-skinned or obese patients.
Multi-functional Integrated Devices
Direction:
Venous imaging + ultrasound.
Combined with hemoglobin monitoring (suitable for dialysis centers).
Contactless Technology
Trend:
Thermal imaging + AI analysis of blood flow abnormalities (early screening for venous thrombosis).
IV. Business Model Innovation
Leasing and Subscription Model
Model:
Hospitals pay per puncture (e.g., $1–2 per puncture), lowering the procurement threshold.
Device manufacturers generate ongoing revenue through consumables (e.g., disinfectant sleeves).
Lower-tier market channels
V. Policy and Capital Trends
Policy Benefits
China’s 14th Five-Year Plan for medical equipment explicitly supports imaging equipment for grassroots healthcare facilities.
The FDA’s 2023 regulations simplify the approval process for portable imaging devices.
Investment Hotspots
Startup directions:
Focus on niche scenarios (e.g., VeinTech’s pediatric-specific devices).
Wearable devices (ring-shaped imaging devices have attracted attention from Silicon Valley venture capitalists).
Also welcome to contact us, we are ZD Medical Inc.
Tel : +86-187 9586 9515
Email : sales@zd-med.com
Whatsapp/Mobile : +86-187 9586 9515