In the critical field of biological specimen transportation, "selecting the correct packaging" serves as the primary line of defense to ensure sample integrity and regulatory compliance. Different scenarios present unique challenges: hospitals fear "wasting space with large bags, or insufficient space with small ones" for blood samples; clinical research demands stringent separation to prevent "cross-contamination" among multiple specimens; and international shipping risks "non-compliance detentions by customs."
To address the core needs of these diverse applications, AIC's specialized transport bag series, including the ai650, L300420, and APS4X320, are each strategically designed. This article will provide a precise needs-matching solution through a "Scenario-Based Selection Guide + Parameter Comparison + Customization Options," helping you accurately select models and avoid common pitfalls.
Scenario-Based Selection: Prioritize Transport Needs,Then Match the Model
1. Hospitals / Health Check Centers: Short-Haul Blood/Urine Specimen Transport — Prioritize "Small Size + High Transparency"
Core Needs: Small sample volume per trip (typically 1-2 test tubes), short transport distance (inter-departmental or intra-city), and the need for rapid specimen information verification (to avoid repeated opening of the package).
Recommended Model: 6x9 inch 95kPa Blood Specimen Transport Bag (A standard variant of the ai650 Series)

Why Choose This?
Optimal Size: The 6x9 inch (approx. 15 cm×23 cm) size perfectly accommodates 1-2 standard 5−10 ml blood collection tubes. The compact bag is lightweight, easily carried by medical staff, or fits into a care kit, preventing "oversized packaging" which can lead to sample agitation.
Material Advantage: Made from high-clarity Polyethylene (PE) material, it allows for visual verification of patient information and specimen ID on the tube label without opening the package, reducing the frequency of openings and minimizing contamination risks.
Basic Protection: Compliant with UN3373 and PI650 standards, its 95kPa pressure resistance handles common intra-city transit jolts (e.g., ambulance braking, elevator movement). Paired with a thin integrated absorbent layer, it can contain small amounts of condensation or minor accidental fluid leaks.
2.Clinical Research Institutions: Multi-Specimen / Multi-Site Transfer — Essential: "Compartmentalized Absorbent Bags"
Core Needs: Transportation of multiple specimens in a single shipment (e.g., 4-7 blood/CSF samples from different subjects), the requirement for strict segregation to prevent cross-contamination, and a robust defense against "leakage in the event of sample damage."
Recommended Model: APS4X320 4/7 Compartment Absorbent Bag (Used in conjunction with the ai650 Outer Packaging Bag)
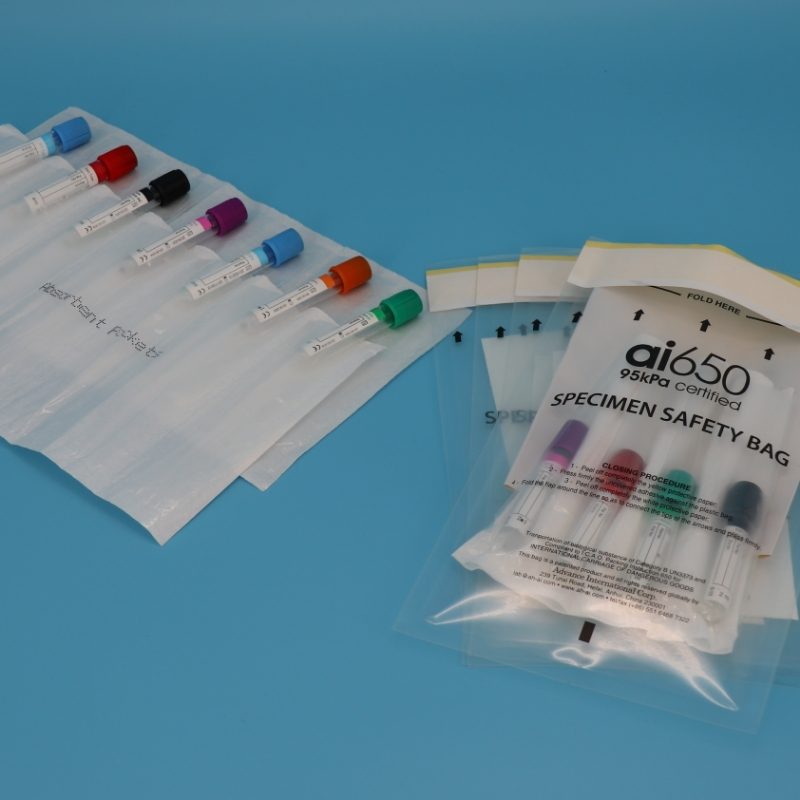
Why Choose This?
Physical Segregation: The bag features 4 or 7 independent retention slots (card slots), each securing 1 test tube or small vial. This complete separation prevents tube collision during transport, thus avoiding label abrasion and specimen mixing.
Superior Absorption: The absorbent layer within the slots utilizes Superabsorbent Polymer (SAP), capable of absorbing up to 50 ml of liquid per slot (equivalent to the full volume of a standard test tube). Should a primary receptacle break due to impact, the leaked fluid is instantly immobilized, preventing wicking to other slots or the outer bag.
Broad Compatibility: It accommodates not only standard blood collection tubes but also 15 ml centrifuge tubes and 20 ml reagent vials, making it ideal for clinical studies requiring the "transfer of multiple specimen types in one batch."
3. International Biopharma / Cross-Border Labs: Long-Haul Air / Sea Freight — Essential: "Fully Compliant ai650 Series"
Core Needs: Passing UN3373 compliance checks by customs in regions like Europe, the Americas, and Southeast Asia; withstanding pressure changes during long-haul transport (e.g., high-altitude low-pressure environments in air freight); and ensuring long-term preservation of specimen labels and documentation.
Recommended Model: ai650 95kPa Certified Model (Can be paired with a separate Document Pouch)

Why Choose This?
Full Compliance: The bag is pre-printed with the UN3373 Biohazard Mark and the 95kPa Pressure Certification Seal. It comes with a manufacturer's Certificate of Compliance (ready for submission to customs). This simplifies adherence to international regulations such as the IATA DGR (Dangerous Goods Regulations) and US DOT rules, preventing delays or detentions due to "incomplete labeling."
Pressure and Stress Resistance: Subjected to simulated air transport pressure tests (repeated pressurization from −50 kPa to 95 kPa), the bag demonstrated no rupture or leakage. It is built to withstand the rigorous conditions of long-haul transportation, from China to Europe (approx. 12 hours air freight) or China to Southeast Asia (approx. 3 days sea freight).
Document Protection: Features a separate external document pouch for insertion of the specimen manifest, customs declaration forms, and test reports. This ensures the documentation is completely isolated from the specimens (preventing liquid contamination of documents) and the pouch uses waterproof material, protecting the paperwork even if the outer package encounters rain.
3 Steps to Quickly Identify the "Optimal Solution
Define the Scenario: First, clarify the nature of the transport: "Short-haul / Long-haul," "Single Specimen / Multiple Specimens," and "Standard / Special Environment" (e.g., cryogenic, radiation).
Match Parameters: Compare the scenario against the "Parameter Comparison Table," prioritizing "Regulatory Certification" and "Size" (e.g., international transport must check UN3373, multi-specimen transport must check the compartmentalized design).
Inquire About Customization: If standard models are insufficient (e.g., for extra-low temperature or large volume), directly contact the customization service to avoid using standard bags that introduce risk.
If you are still uncertain about the selection process, please contact the AIC Technical Team at www.aicbiologicalbag.com. Provide your specimen type and transportation details, and we will offer a complimentary "Selection Recommendation Report." You may also request free samples for testing, ensuring an accurate and risk-free choice.